At FUSION GENOMICS, our technology represents a paradigm shift in infectious disease diagnostics. We have harnessed the power of genomics to develop a revolutionary solution that addresses the limitations of traditional diagnostic tests.
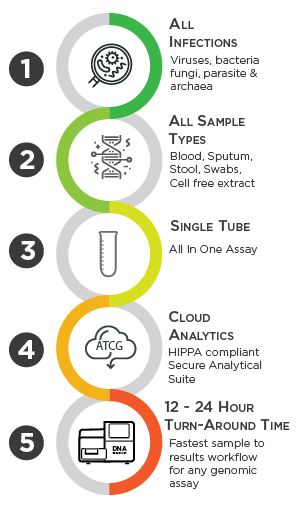
The ONETest™, is a targeted metagenomics assay fully automated using a standard laboratory fluidics station. It utilizes state-of-the-art and patented capture probes, called QuantumProbes and informatics to detect the full range of human pathogens in a single test. This comprehensive approach enables us to provide clinically relevant genomic information for accurate and timely diagnosis.
The ONETest™ workflow begins with the collection of a patient sample, which is then processed using our fully automated workflow. During this process, the ONETest™ enriches the patient sample for DNA fragments belonging to targeted pathogens. This enriched DNA library is then ready to be sequenced using any benchtop DNA sequencer. Our streamlined process ensures rapid turnaround time, with results delivered within 24 hours.
The ONETest™ has undergone rigorous validation studies with global agencies and renowned institutions, including the European CDC, Canadian Food Inspection Agency, University of Florida Health, Seattle Children’s Hospital, Sunnybrook Hospital (Toronto), and Merck Sharp & Dohme. These validations demonstrate the performance, accuracy, and reliability of our technology.